Flomax
"Purchase 0.4mg flomax mastercard, prostate 180 at walmart".
By: R. Gamal, M.B.A., M.B.B.S., M.H.S.
Vice Chair, CUNY School of Medicine
The key point is that despite growing evidence of a role of inflammatory cytokines in many cases prostate seed implant order flomax toronto, osteoarthritis is not always necessarily an inflammatory disorder of the joint prostate infection symptoms order flomax uk. For the July 2009 release prostate 02 flomax 0.2mg low cost, the domain for these attributes was expanded to evaluation procedure mens health fat burning workout buy 0.2 mg flomax with amex. The attributes used to define those concepts when they were descendants of Clinical finding were retained after the concepts were moved to the Event hierarchy. Additional editorial policies for the use of attributes in the Event hierarchy have yet to be established. This hierarchy represents a broad variety of activities, including but not limited to , invasive procedures. Such complex statements should utilize two or more procedure codes that are placed into an appropriately structured information model. The anatomical site may be directly acted on (Procedure site - Direct) or indirectly acted upon (Procedure site - Indirect). Removals of devices, calculi, thrombi, foreign bodies and other non-tissue entities from the structure should use Procedure site - Indirect. An example of an exception to this rule would be removal of a calculus from the ureter. In this case, the calculus is the direct object, but there is no procedure site that is that direct object, since the ureter is an indirect object. Grafts that become attached via in-growth of capillaries, fibroblasts, and/or other cells or tissues would also be regarded as biologically connected, and therefore modeling their removal would include the anatomical structure as a direct object of the action. The anatomical structure is not to be modeled as a direct object of a removal only when the procedure does not necessarily involve removal also of part of the anatomy; examples include removals of things such as a foreign body, a catheter, a renal calculus, or a mechanical implant like a pacemaker. Exceptions (concepts that do not specify a direct object, but only an indirect object) are usually general groupers such as Arm implantation (procedure) (meaning implantation of something into the arm), since the thing implanted could be either a device or a substance (material). It is correct to regard each relationship group as a kind of sub-procedure that defines the overall procedure. Each method can be regarded as the verb of a sentence, and the verbs direct and indirect objects are specified by the site, morphology, device, substance or energy attributes (below) that are grouped with it. These concepts descend from Drug-device combination product (product) which is a descendant of both Device (physical object) and Pharmaceutical / biologic product (product). The mitral valve prosthesis is where the excised vegetation is located but the mitral valve prosthesis itself is not excised. For example, it can be used in blood banking procedures to differentiate whether the procedure was performed on the donor or the recipient of a blood product. It is not used for a procedure where the subject of the procedure is someone other than the subject of record. The domain for this attribute is the sub-hierarchy below Administration of substance via specific route (procedure) 433590000. When the type (body structure, device, or substance) of direct object is indeterminate, the direct-object attributes should not be used. It might be considered to be defined as a procedure done by a physician, but even in this case, it would be deprecated on the basis that it is provider-specific. On the other hand, it seems quite clear (despite some dictionary definitions) that surgical procedures are not defined simply as procedures done by a surgeon; a surgeon can carry out many non-surgical actions (examining patients, prescribing, advising, etc). Even more important, a surgical procedure need not necessarily be performed by a surgeon; if a non-surgeon does a procedure that is surgical, it still remains a surgical procedure. Procedure While there may never be a complete consensus as to what constitutes a surgical procedure, the agreement has been to classify concepts as surgical procedures if their method is a surgical action based on the action hierarchy. In turn, the surgical action hierarchy distinguishes surgical from non-surgical actions based on the working definition above. Note the or in the sentence; actions that do not involve cutting or incision, but do involve the intentional non-transient alteration of anatomy, are still surgical. Examples of non-surgical actions include fine-needle or brush biopsies, phlebotomy, aspiration, and closed reduction of dislocations - since they both do not significantly or non-transiently alter anatomy and do not necessarily involve cutting. Examples of borderline actions that are currently classified as surgical include core needle biopsies - these are more invasive and result in more tissue removal than fine-needle biopsies - and centesis, on the theory that combining puncture with removal alters body structure. Unresolved issue: fine-needle biopsy could be viewed as a kind of centesis, but the former is non-surgical and the latter is surgical.
This product contains only antibody to respiratory syncytial virus and does not interfere with the immune response to licensed live or inactivated vaccines prostate meme flomax 0.2mg free shipping. Inactivated Vaccines Antibody-containing products interact less with inactivated prostate cancer 20s order genuine flomax on-line, recombinant subunit prostate oncology 360 0.4 mg flomax visa, and polysaccharide vaccines and toxoids than with live vaccines (73) prostate cancer questionnaire purchase 0.2mg flomax otc. Therefore, administering inactivated vaccines and toxoids either simultaneously with or at any interval before or after receipt of an antibody-containing product should not substantially impair development of a protective antibody response (Table 3-4). The vaccine or toxoid and antibody preparation should be administered at different sites using the standard recommended dose. General Best Practice Guidelines for Immunization: Timing and Spacing of Immunobiologics 24 Interchangeability of Single-Component Vaccines from Different Manufacturers Certain vaccines that provide protection from the same diseases are available from different manufacturers, and these vaccines usually are not identical in antigen content or in amount or method of formulation. Manufacturers use different production processes, and their products might contain different concentrations of antigen per dose or a different stabilizer or preservative. Available data indicate that infants who receive sequential doses of different Hib conjugate, hepatitis B, and hepatitis A vaccines produce a satisfactory antibody response after a complete primary series (74-77). All brands of Hib conjugate, hepatitis B,(d) hepatitis A, rotavirus,(e) and quadrivalent meningococcal conjugate vaccines are interchangeable within their respective series. If different brands of a particular vaccine require a different number of doses for series completion. For Hib vaccines, any monovalent or combination conjugate vaccine is acceptable for the booster dose of the series, if only one product was used for the primary series (56). Limited data are available about the safety, immunogenicity, and efficacy of using acellular pertussis. However, in the absence of a clear serologic correlate of protection for pertussis, the relevance of these immunogenicity data for protection against pertussis is unknown. In a postlicensure study, meningococcal conjugate vaccines from different manufacturers were evaluated for successive doses of meningococcal conjugate vaccine. For vaccines in general, vaccination should not be deferred because the brand used for previous doses is not available or is unknown (29,79). Unknown or Uncertain Vaccination Status Vaccination providers frequently encounter persons who do not have adequate documentation of vaccinations. The rationale for acceptance for influenza vaccine is that the time period of recall is one year or less, making it very likely that correct recall will occur. Serologic General Best Practice Guidelines for Immunization: Timing and Spacing of Immunobiologics 26 testing for immunity is an alternative to vaccination for certain antigens. However, commercial serologic testing might not always be sufficiently sensitive or standardized for detection of vaccine-induced immunity (with the exception of hepatitis B vaccination at 1-2 months after the final dose), and research laboratory testing might not be readily available. However, doses administered at ages <12 months should not be counted as part of the series (81). Use of licensed combination vaccines is generally preferred to separate injections of their equivalent component vaccines. When administering combination vaccines, the minimum age for administration is the oldest age for any of the individual components. The minimum interval between doses is equal to the greatest interval of any of the individual components. Information on other vaccines that are licensed in the United States but not distributed, including anthrax and smallpox, is available at emergency. These vaccines should not be administered to infants aged <6 weeks because of the other vaccine components. If the second dose is given less than five months after the first dose, but more than four weeks after the first dose, the next dose should be administered at least 12 weeks after the second dose, and at least 6-12 months after the first dose. If the third dose was administered on or after December 16, 2016, and was administered 12 weeks after the 2nd dose and 5 months after the first dose, it is a valid dose. If the third dose was administered before December 16, 2016, and was administered 12 weeks after the 2nd dose, and 16 weeks after the first dose, it is a valid dose. If the third dose was administered on or after December 16, 2016, and was administered. To determine which children younger than 9 years should receive 2 doses in a single season, please see influenza vaccine-specific recommendations (82).
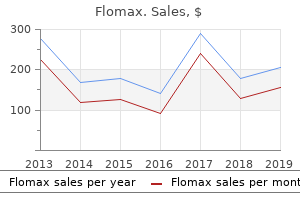
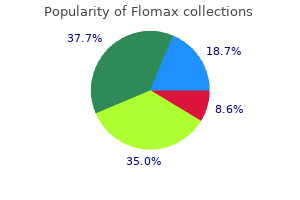
However is it fair that after spending millions they have an authorized generic to deal with prostate oncology marina del rey purchase 0.2mg flomax otc. One such report androgen hormone in females buy flomax mastercard, while indicating that "authorized generics have eroded a significant financial incentive for the industry prostate cancer signs purchase generic flomax online,"59 nevertheless projected substantial profits from the exclusivity opportunities studied "even after adjusting for likely competition from authorized generics androgen hormone foods buy flomax canada. In the next chapter we examine the quantitative data for further insights into their long-term competitive effects. Shapiro, the Impact of Authorized Generic Pharmaceuticals on the Introduction of Other Generic Pharmaceuticals (2007). With respect to the latter point, the authors present their own quantitative analysis, concluding that "competition from authorized generics does not reduce R&D by generic manufacturers, and therefore should not reduce or delay the introduction of future generic products. The analysis in this chapter is based on two samples of market outcomes: one drawn from drugs for which an exclusivity period was granted but has expired and another based on drugs for which no exclusivity was ever granted. These two scenarios will collectively be referred to as non-exclusivity situations. In contrast, the number of manufacturers actively selling a generic version of a drug can range from one to more than fifteen outside of exclusivity. This chapter presents similar analysis using market data from non-exclusivity situations. This first-mover advantage seems to be an important benefit of introducing a product during the exclusivity period. It next discusses data sources and analytical methodology, explaining that the data derive from the same sources as in Chapter 3 but that the analysis needs to be tailored to account for differences between exclusivity and non-exclusivity periods. Prior Studies As summarized below, two studies examined the impact of additional generic competitors on price levels after the 180-day exclusivity period. Consistent with earlier literature, the authors discover that at 24 months after generic entry ". This is consistent with another study that addresses this issue and concludes that the introduction of authorized generics is "least problematic. Data and Methodological Approach the data analysis presented below has two main goals. In the first instance, the period of study begins immediately following expiration of the exclusivity period (typically six months after initial entry), and includes the same set of products investigated in Chapter 3. Because of sample attrition over time, only the first 36 months following generic entry are included in the sample used for the analysis in this chapter. The analysis outside of exclusivity controls for the number of generic manufacturers providing the product. Table 6-1 summarizes some key statistics related to this variable outside of exclusivity. For example, markets with a single manufacturer are the most commonly observed competitive arrangement in the data, accounting for approximately 22% of observations. Each product accounts for a number of observations equal to the number of months the product is observed in the data, so the results reported may differ substantially from a listing that combined all observations for the same drug. For instance, suppose 600mg Gabapentin tablets account for an observation in the three-manufacturer row of Table 6-1 in a particular month. Then if another manufacturer enters, that product accounts for one of the observations in the four-manufacturer row for the next month. If the number of manufacturers stays at four for several months, each of those months counts as another observation in the four-manufacturer row. In some of those months, there are also four manufacturers selling 800mg Gabapentin tablets, which also would be counted in the fourmanufacturer row. The measure of price in these figures is the average wholesale generic relative price, derived, as in Chapter 3, by dividing the generic price by the average price of the branded version of the product in the three months preceding generic entry. The price decline observed over this period occurs alongside a fairly steady increase in the number of manufacturers over time.
This product is designed for researchers involved in studying processes influenced by steroid hormones such as estrogen stimulation and the obesity process and other areas that benefit from reduced hormone levels such as certain types of viral infections prostate infection generic 0.2mg flomax amex. Please check with your local supplier regarding availability and import restrictions in your country prostate cancer urologist vs oncologist 0.4mg flomax. Heat inactivation also inactivates other undetermined inhibitors of cell growth in culture prostate 32 buy cheap flomax. Development of a more rapid mens health best flomax 0.4mg without a prescription, reduced serum culture system for Caco-2 monolayers and application to the biopharmaceutics Each lot is supplemented to a constant iron concentration. For example, it is used in cornea preservation and cryopreservation of bovine embryos. Nevertheless, products of human origin should be considered potentially infectious and handled accordingly. Contains highly purified, heattreated bovine serum albumin, heat-treated bovine transferrin and bovine insulin. Does not contain growth factors, steroid hormones, glucocorticoids, cell adhesion factors, detectable Ig and mitogens. Serum Replacement 1 Useful for the study of the effects of growth factors or the production of biologicals (viruses, enzymes, monoclonal antibodies). Serum Replacement 2 Contains additional components necessary for cells requiring higher concentrations of serum. Serum Replacement 3 Designed primarily for the growth of human cells and the production of proteins. Product should still be considered potentially infectious and handled accordingly. Contains highly purified, heat-treated bovine serum albumin, heat-treated bovine transferrin and bovine insulin. In 1885, Sydney Ringer developed a solution of inorganic salts designed to maintain contractility of mammalian heart tissue. A less specific salt solution was designed by Tyrode for use in work with primary mammalian cells. Tyrodes salt solution became the accepted fluid for diluting protein components of media of natural origin. Since that time, many other balanced salt solutions have been developed for use in cell and tissue culture. The current role of a balanced salt solution in cell culture is multifaceted and can be divided into four principal functions: serves as an irrigating, transporting and diluting fluid while maintaining intra- and extracellular osmotic balance; provides cells with water and certain bulk inorganic ions essential for normal cell metabolism; combined with a carbohydrate, such as glucose, provides the principal energy source for cell metabolism; and provides a buffering system to maintain the medium within the physiological pH range (7. Use this medium when working with stem cells or when growing cells at low densities. Phenol red has been shown to interfere with the growth of some cells at cloning densities. It is routinely used as an anti-coagulant/ blood preservative, which permits the storage of whole blood at refrigerator temperatures for approximately 10 weeks. With sodium bicarbonate, liquid, sterile-filtered, suitable for cell culture endotoxin. Accelerate your research and create permanent gene knockouts with CompoZr Knockout Zinc Finger Nucleases from Sigma Life Science. This testing is designed to eliminate the need for screening biochemicals prior to use in a cell culture application. Biological Performance Protocol Sigmas reagents and supplements are evaluated for their ability to promote or maintain cell growth while demonstrating no cytotoxic effects. Biochemicals that are used as medium components are added to a basal medium at a concentration consistent with their biochemical activity or nutritional importance. The medium is supplemented with 10% fetal bovine serum, and growth performance is assessed using appropriate cell lines. The cell lines chosen represent a cross section of cells with a broad spectrum of nutritional requirements.
Flomax 0.2mg line. Ross Butler Shows His Gym & Fridge | Gym & Fridge | Men's Health.